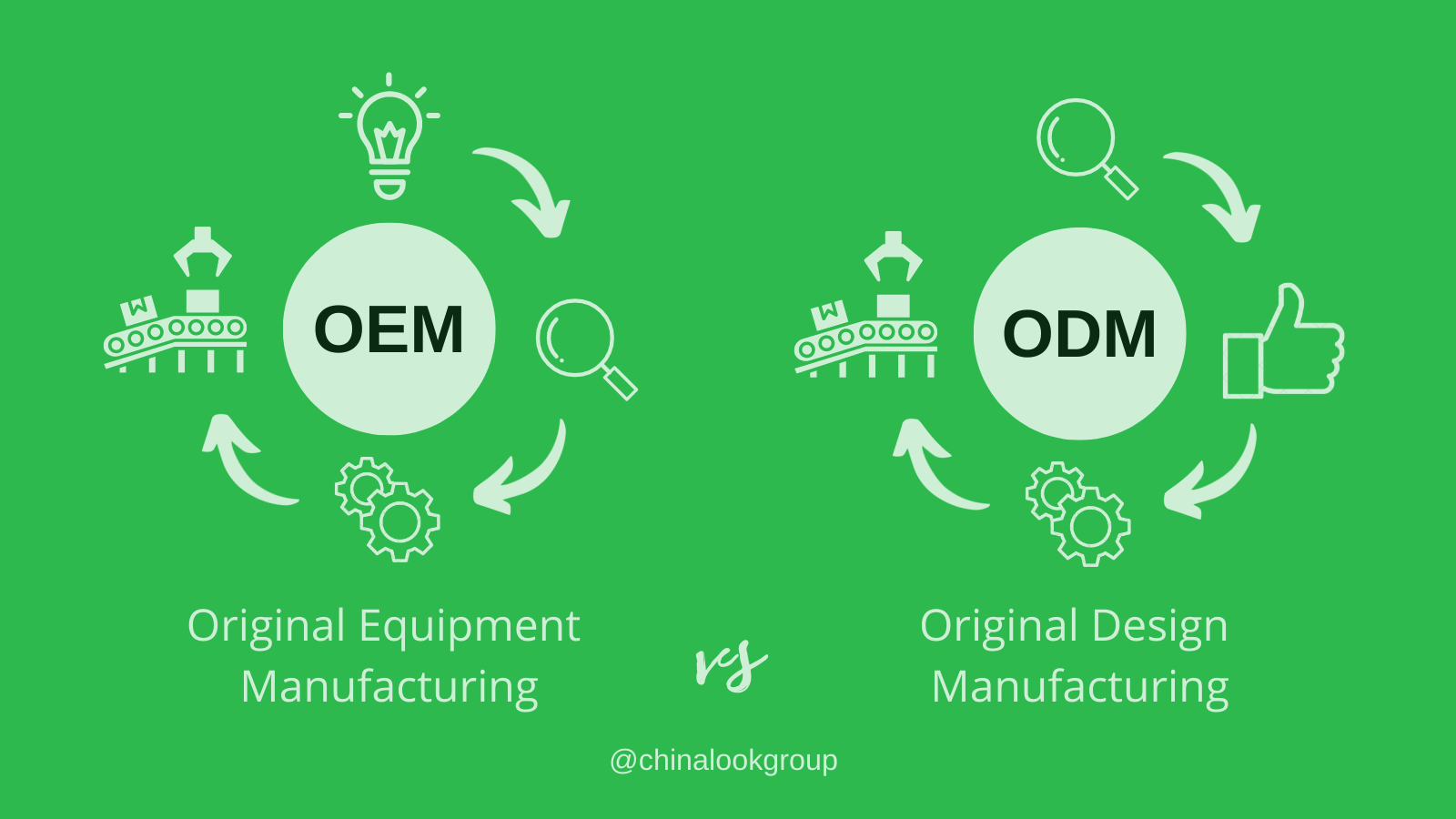
OEM/ODM Services
1. What’s the difference between OEM and ODM services?
-
OEM (Original Equipment Manufacturer): You provide the specifications; we manufacture the product with your brand.
-
ODM (Original Design Manufacturer): You share your concept or functional requirements; we handle the product design, development, and manufacturing.
2. What types of medical products do you offer OEM/ODM services for?
We specialize in:
-
Cardiovascular devices (e.g., inflation devices, catheters, stents)
-
Interventional accessories
-
Diagnostic kits
-
Home care or portable monitoring tools (on a case-by-case basis)
3. What is your typical OEM/ODM development process?
-
Requirement collection
-
Feasibility & quotation
-
Design and prototype development
-
Validation and regulatory support
-
Mass production and delivery
We maintain strict confidentiality and IP protection throughout.
4. Do you support small volume OEM orders?
Yes. We support both pilot-scale and large-scale production, especially for startups or distributors validating a new market.
5. Can you help with product registration and certification (CE, FDA, etc.)?
Yes. We can provide technical documentation, biocompatibility data, and manufacturing certifications to support your registration needs. We can also connect you with CRO and regulatory partners if needed.
6. What is your typical lead time for OEM/ODM projects?
-
Concept to prototype: 4–8 weeks
-
Prototype to mass production: 8–12 weeks (depending on complexity and regulatory needs)
7. What packaging and branding options do you offer?
We offer:
-
Custom packaging design (box, pouch, labels)
-
Brand printing on products
-
IFU and manual customization
-
Language localization
8. Is my idea safe with your company?
Absolutely. We sign NDA (Non-Disclosure Agreements) and confidentiality clauses for every OEM/ODM collaboration. Your IP and design are protected.
 Peripheral Aspiration Catheter
Peripheral Aspiration Catheter
 Inflation Device
Inflation Device