FastWave Medical to commence coronary feasibility study of laser IVL system
FastWave Medical has secured institutional review board (IRB) approval to commence a coronary feasibility study utilising Sola, its novel laser intravascular lithotripsy (L-IVL) system. FastWave is partnering with Clinical Accelerator to achieve this milestone, paving the way for its planned US pivotal trial.
FastWave’s next-generation Sola L-IVL system empowers physicians treating cardiovascular calcium with sleek, rupture-resistant balloon catheters.
The Sola platform allows for improved operator control to safely and effectively modify calcium, FastWave says in a press release, using a custom laser energy source that produces actuating, circumferential sonic pressure waves.
“FastWave’s laser IVL system is sophisticated, yet very easy to use. It holds a lot of promise for reshaping complex arterial disease treatment, enabling cardiologists to treat calcified arteries with more precision and efficiency,” Amir Kaki (Henry Ford St John Hospital, Detroit, USA).
“Our committed team is aiming to set a new standard in the IVL category, enabling physicians to achieve best-in-class clinical outcomes. This IRB approval represents a major milestone in our mission to deliver transformative technology to physicians and their patients,” said Scott Nelson, co-founder and CEO of FastWave.
In addition to this coronary feasibility study, FastWave has plans to commence a US investigational device exemption (IDE) pivotal trial this year for the treatment of peripheral artery disease with its Artero electric IVL (E-IVL) system.
“FastWave is taking the reins and rapidly advancing IVL therapy with its differentiated peripheral and coronary systems. On behalf of my fellow interventionalists, we’re genuinely excited to have more IVL options to treat patients with complex arterial disease,” said Art Lee, director of Peripheral Vascular Services at The Cardiac & Vascular Institute (TCAVI, Gainesville, USA).
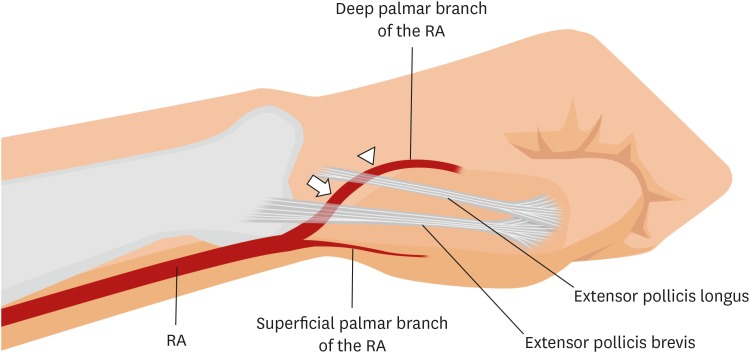 About Radial Access – Compression Tourniquet
About Radial Access – Compression Tourniquet
 Mechanical Venous Thrombectomy Using Indigo Aspiration System: A Case Report
Mechanical Venous Thrombectomy Using Indigo Aspiration System: A Case Report
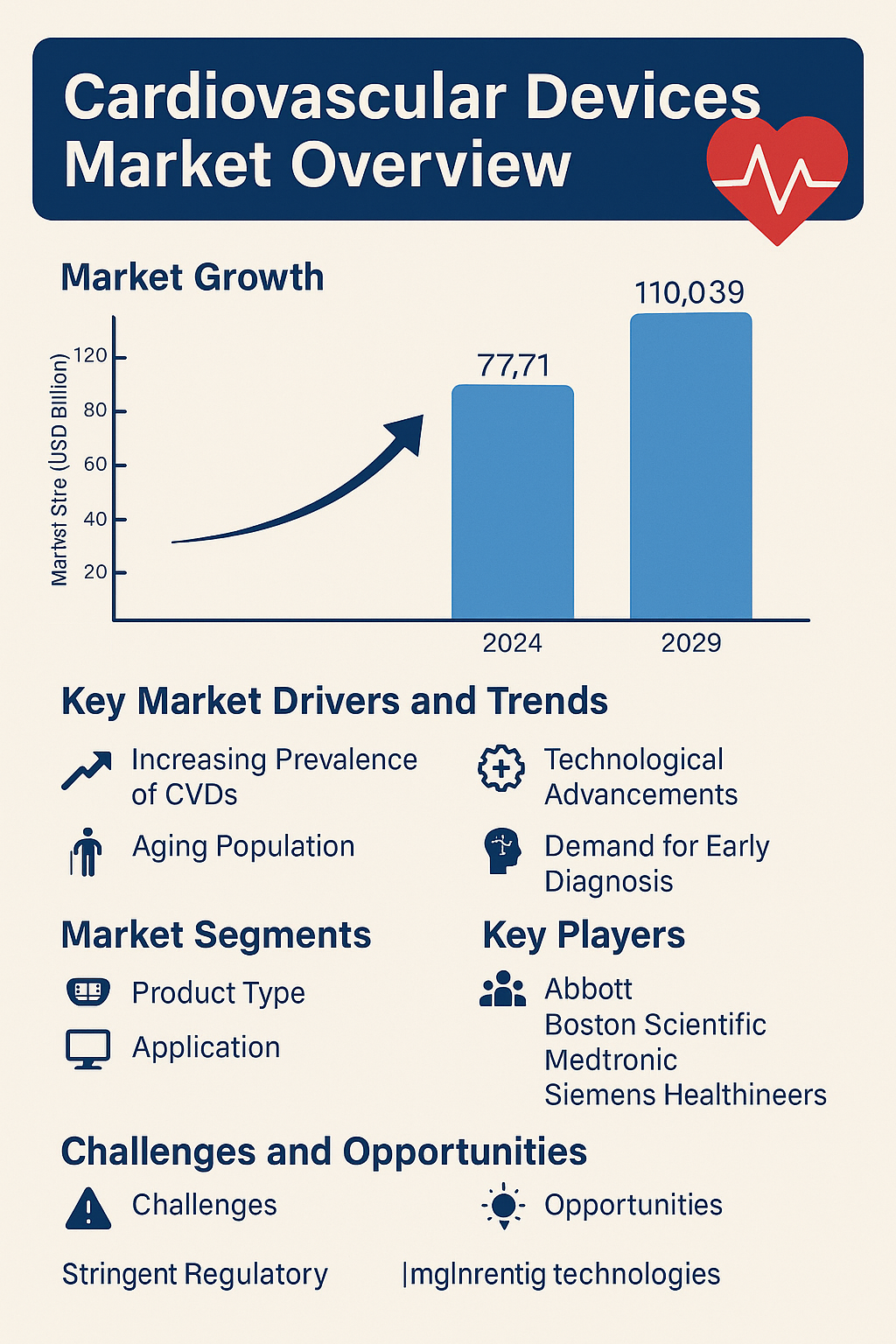 Global market for cardiovascular devices
Global market for cardiovascular devices
 SCAI names Srihari S Naidu as president
SCAI names Srihari S Naidu as president



