Our Inflation Device Receives KFDA Approval and Earns Positive Market Feedback in Korea
We are proud to announce that our Inflation Device Kit has officially received KFDA (Korea Food and Drug Administration) approval, marking a significant milestone in our global regulatory journey. This approval confirms that our product meets Korea’s stringent medical device safety and performance standards.
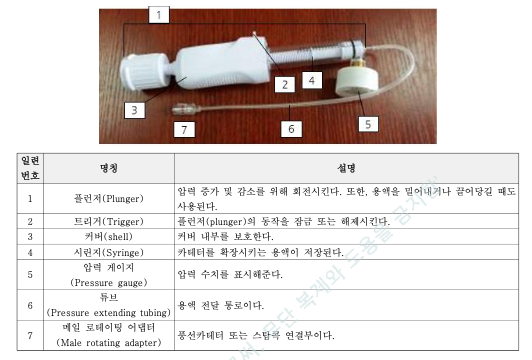
Since its launch in the Korean market, our inflation device has been successfully adopted by local hospitals and interventional cardiologists, with consistently positive feedback on both product reliability and ease of use. Clinicians have praised its:
-
Precise pressure control (30 atm & 40 atm options)
-
Ergonomic design and intuitive handling
-
High durability and stability during procedures
The Korean approval adds to our growing list of regulatory successes, further validating our commitment to delivering safe, high-quality, and innovative interventional tools to medical professionals around the world.
We deeply appreciate the trust from our Korean partners and end users, and we will continue to support clinical teams with high-performance solutions.
 Successful PMDA Registration: Our Inflation Device Enters the Japanese Market
Successful PMDA Registration: Our Inflation Device Enters the Japanese Market
 Peripheral Aspiration Catheter
Peripheral Aspiration Catheter
 Peripheral Aspiration Catheter
Peripheral Aspiration Catheter



