Successful PMDA Registration: Our Inflation Device Enters the Japanese Market
We are excited to share that our Inflation Device Kit has officially obtained PMDA (Pharmaceuticals and Medical Devices Agency) registration in Japan, one of the world’s most regulated and quality-driven medical markets.
This achievement reflects our commitment to:
-
Meeting global standards for safety, quality, and clinical effectiveness
-
Investing in regulatory excellence to expand our global footprint
-
Bringing trusted cardiovascular tools to healthcare professionals worldwide
With PMDA registration now complete, our inflation devices are being introduced to select Japanese hospitals and distributors. Initial responses from clinicians emphasize:
-
High accuracy and pressure control
-
Superior build quality and ergonomics
-
Smooth performance under demanding cath lab environments
We’re proud to contribute to Japan’s advanced interventional ecosystem and to support local physicians in delivering outstanding cardiovascular care.
 Peripheral Aspiration Catheter
Peripheral Aspiration Catheter
 Peripheral Aspiration Catheter
Peripheral Aspiration Catheter
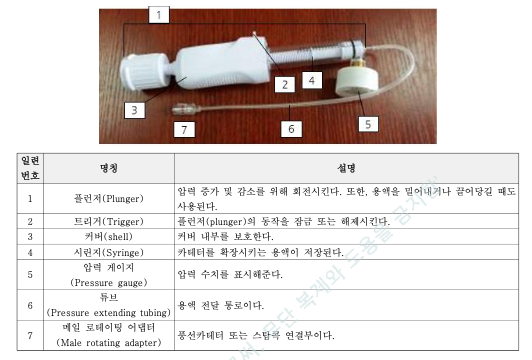 Our Inflation Device Receives KFDA Approval and Earns Positive Market Feedback in Korea
Our Inflation Device Receives KFDA Approval and Earns Positive Market Feedback in Korea



